Crispr/Cas9 is working well…but we’re still learning how to move this cargo into specific cells. (2/3)
Hello friends,
I’m Kevin Curran, a molecular biologist and life science educator. The latest posts in my newsletter are focused on the delivery of gene editing technology into the intended tissues within the human body.
Solving for gene editing delivery is one of the critical challenges in biotech today. We have developed the capacity to precisely edit DNA, now we need to master the art of deploying this cargo into the right cells in a patient’s body.
In the previous newsletter, I described how viral vectors and ‘naked cargo’ can be delivered into cells. In this post, I review the use of LNPs to reach liver cells and hopefully also non-liver cells.
If you’ve received this email, then you either subscribed or someone forwarded it to you. If the latter is the case, please subscribe by pressing this button:
LNPs are tiny balls of synthetic fat. Various biotech companies have formulated their own proprietary LNPs and we’ve seen already seen a global success with this delivery system in the form of the Pfizer and Moderna Covid vaccines.
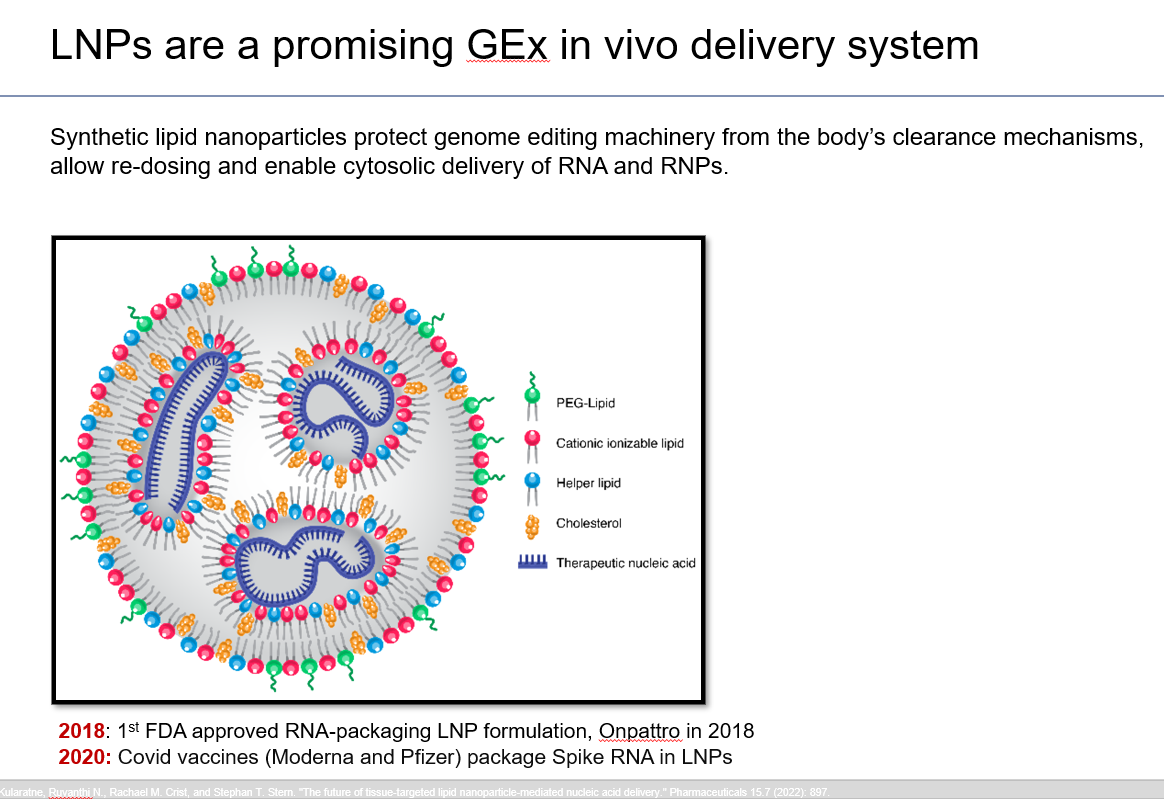
In 2024, biotech companies are relying on LNPs to deliver Crispr/Cas9 into liver cells to address genetic disorders. These efforts are still in the clinical stage. The only commercialized gene editing medicine is performed outside of the human body and relies on electricity to push gene editing cargo into the intended cells.
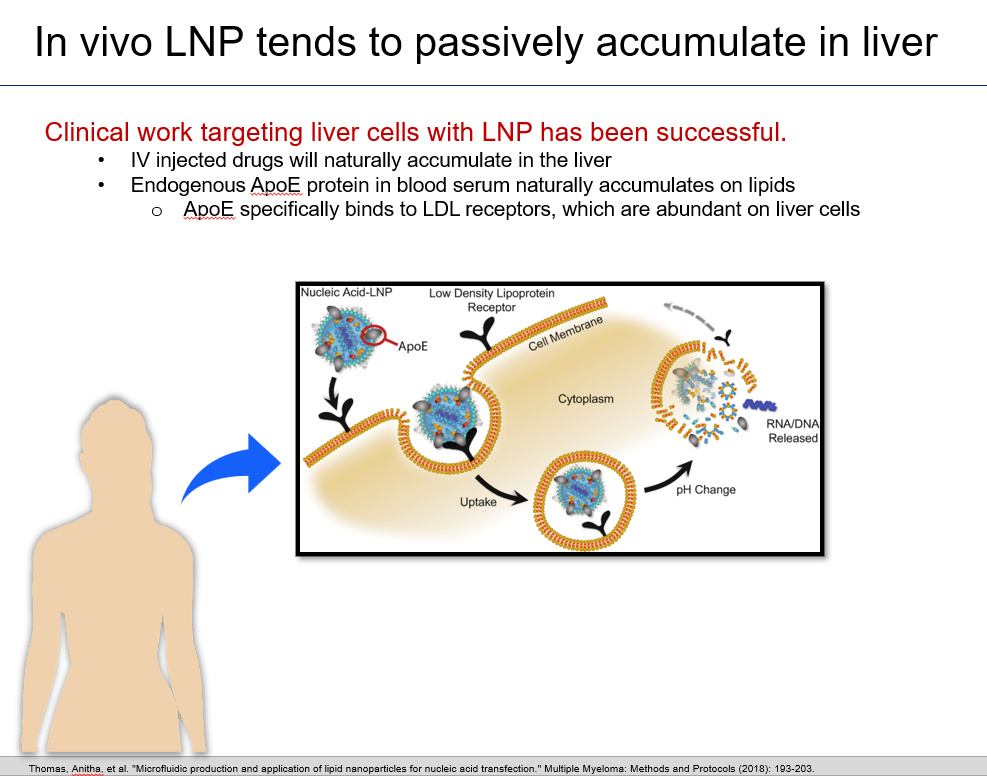
LNPs are naturally suited for in vivo delivery into liver cells. In fact, you could consider delivery into liver cells as the default mode for LNPs.
Intellia is taking advantage of this default mode with NTLA-2001 as they target toxic TTR genes in liver cells.
In the video below, I review the basics of LNPs and describe their use in gene editing therapies. If these videos are helpful, then please let me know and I’ll continue to post them on You Tube and include a link in these newsletters.
Thanks,
Kevin

