The science has turned a corner but the financial side remains murky and difficult.
Folks,
Here we are…labor day has come and gone.
Down here in San Diego, the passing of labor day means we have arrived at the midpoint for our summer season. It’s 99 degrees today, a dry heat, but my avocado trees are feeling it. A late summer rainstorm would be much appreciated.
This Friday, I will be teaching a one hour seminar on the commercial landscape for gene editing medicine. This course is presented by Caring Cross, a non-profit focused on global access to genetic medicine. Join us for the live presentation at 3pm ET.
Click this link to sign up for the free course.
Casgevy is the only gene editing drug available on commercial markets. I will present some early commercial information that Vertex has released. Then I will explore the challenges for broad patient access to this therapy.
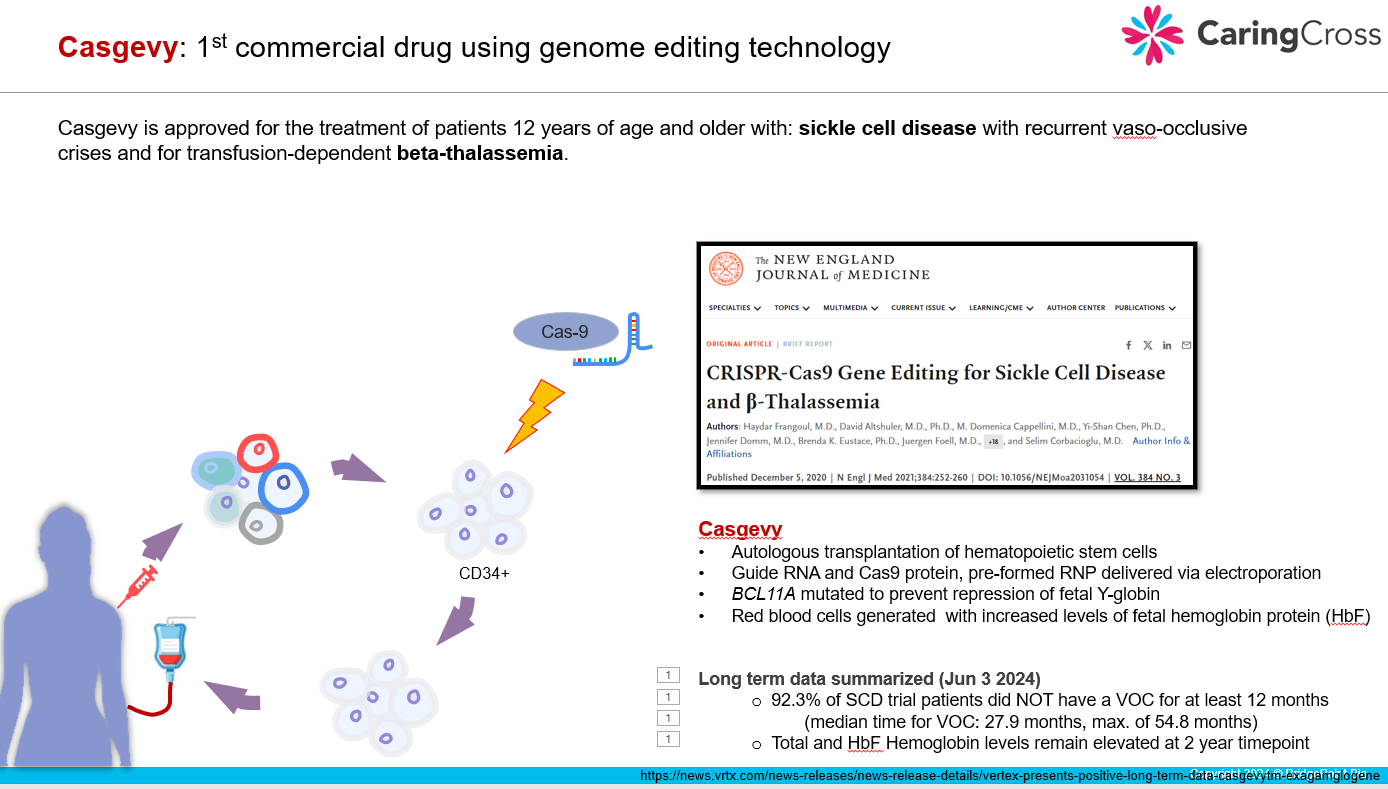
The reality is that our current healthcare system is not set up for broad, global access to Casgevy. We can point the finger at the prohibitive amounts of medical infrastructure required to administer an ex vivo gene therapy. But, the truth is, the world is also lacking a pharmaceutical market structure that allows high priced genetic medicine to readily distribute into low and middle income nations.
As it stands today, high priced genetic medicine can barely distribute into high income nations. Don’t believe me? Ask BioMarin how things are going with Roctavian.
So… something has to change.
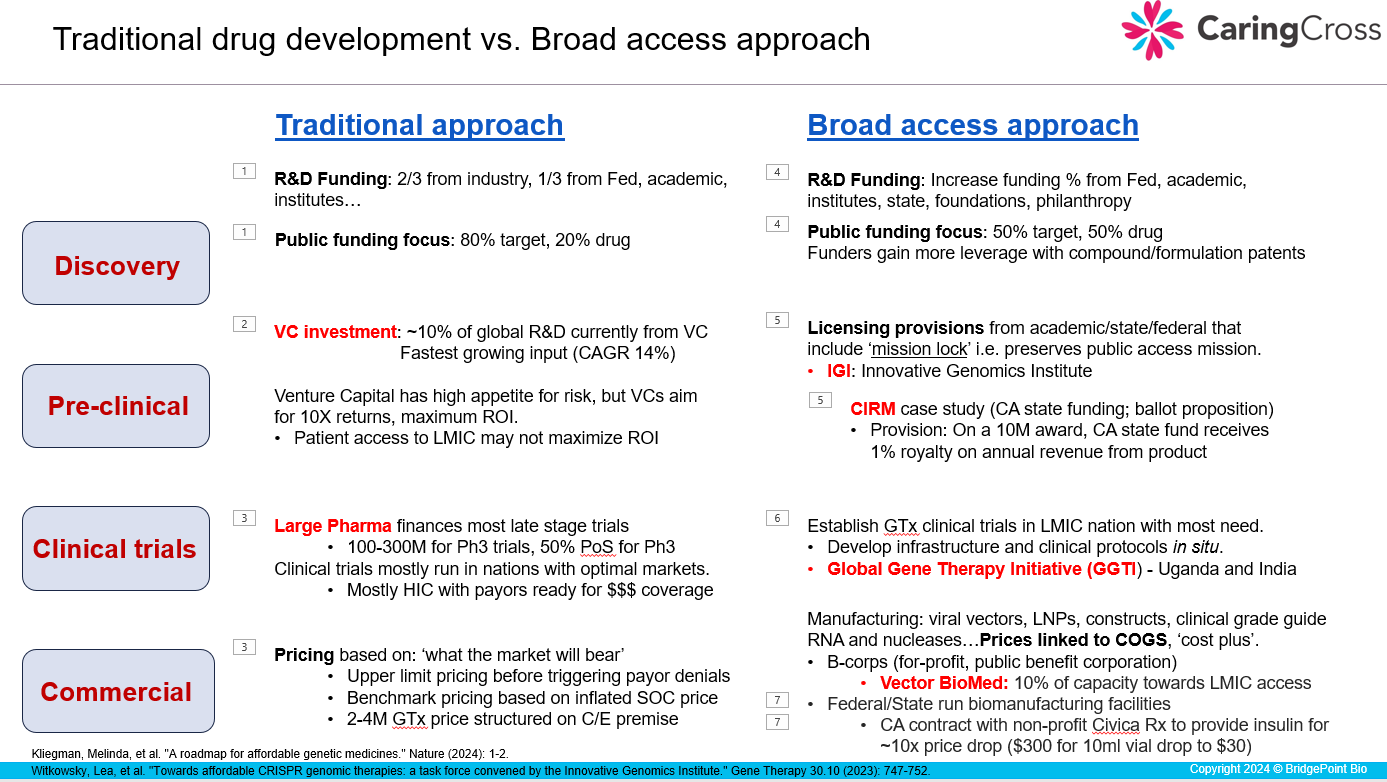
In the image below, I’ve summarized a new approach to drug development. During the course this Friday, I will juxtapose the traditional approach with this Broad Access approach.
Please join us at 3pm ET for this live zoom course. Here’s the link one more time.
https://caringcross.org/events/
Please share this post with the button below…
and… Subscribe to my YouTube channel.
Thanks!
