Lenmeldy therapy genetically engineers bone marrow cells… these cells then travel to the brain to resolve a genetic disorder
This week, the FDA approved a gene therapy for a disease called MLD (metachromatic leukodystrophy). Orchard Therapeutics developed the drug and just reported they will charge $4.25 million for the treatment, making it the most expensive drug in history.
That price tag has been dominating headlines and kicking off a discussion of value based pricing, patient access and the economics of rare disease therapies. Those topics are incredibly important, but in the process of focusing on financing, the scientific advancements leading to this clinical success are ignored.
Below, I briefly explain why the science behind this therapy is so remarkable.
Backstory: We have already seen clinical success removing bone marrow stem cells from the body, genetically engineering them, then infusing them back into the bloodstream and allowing the engineered cells to resolve genetic disorders related to blood cells.
See image below.
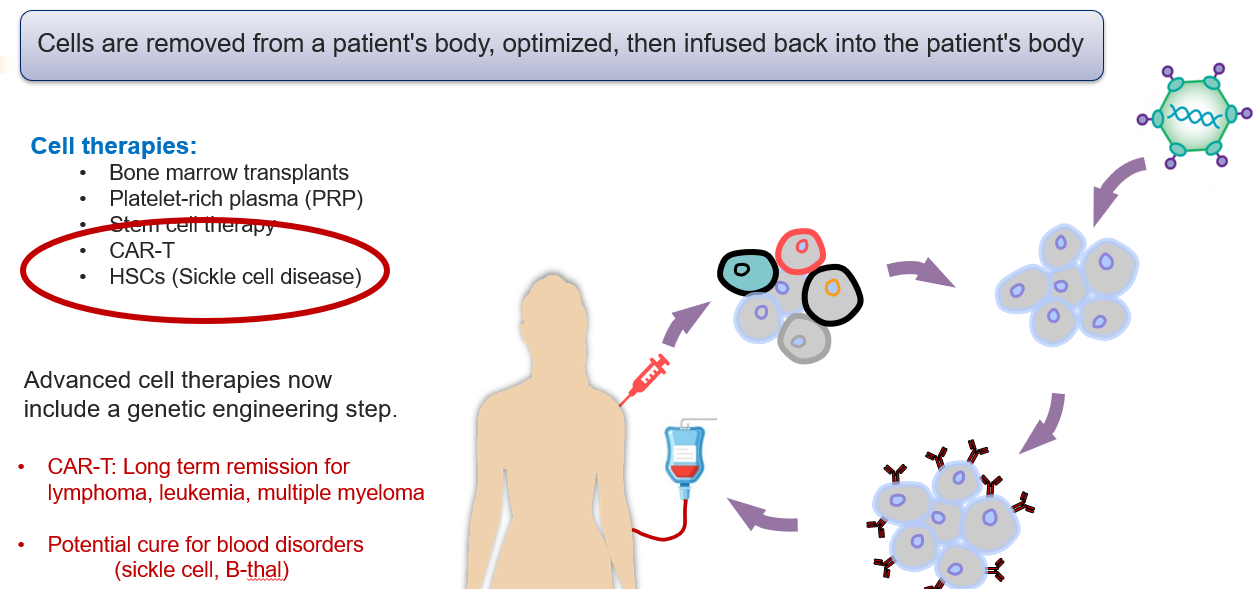
Earlier this year, Vertex and Bluebird Bio both received FDA approval for the approach described above. Both companies are targeting Sickle Cell Disease, which is a genetic disorder of the red blood cells.
Why is the science behind Lenmeldy so remarkable?
Lenmeldy relies on a similar cell delivery strategy, however this new gene therapy targets cells in our nervous system.
MLD disease results from genetic mutations that disable the production of an enzyme called ARSA. Without it, toxic levels of fatty acids build up in the brain and peripheral nervous system, stripping neurons of their myelin sheath. This disorder causes loss of motor function and can be lethal. In order to resolve this disease, we need to deliver ARSA enzymes into the brain and peripheral nervous system.
Lenmeldy relies on a similar cell therapy approach described above, however once the engineered cells are infused into the patient’s bloodstream, the cells then migrate across the blood-brain barrier and engage with tissue in the brain and central nervous system.
This delivery system opens the door for future bone marrow gene therapies to be applied towards genetic disorders of the central nervous system.
See image below to appreciate the challenge of crossing the blood brain barrier. A tight layer of endothelial cells separates our bloodstream from out brain tissue.
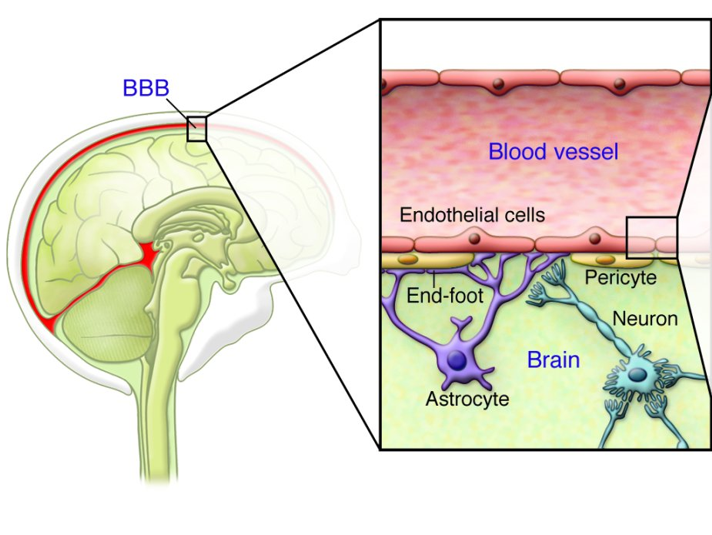
More detail here from Orchard regarding how their genetically engineered bone marrow stem cells (HSPCs) were able to cross the blood brain barrier and deliver ARSA enzyme to brain cells. Quote is from Lenmeldy’s prescribing information
A subpopulation of the infused HSPCs and/or their myeloid progeny is able to migrate across the blood brain barrier to the brain and engraft as central nervous system (CNS) resident microglia and perivascular CNS macrophages as well as endoneural macrophages in the peripheral nervous system (PNS). These genetically modified cells can produce and secrete the functional ARSA enzyme, which can be taken up by surrounding cells, a process known as cross-correction…
So, in summary…. bone marrow stem cells are removed from the blood, then engineered with a lentiviral vector so that they produce large amounts of ARSA enzyme… the cells are infused back into the patient’s blood…. the engineered bone marrow cells develop into microglia and macrophage cells, which can migrate into the brain and secrete ARSA enzyme*… the secreted ARSA enzyme is then internalized by the defunct cells in the brain… and this is all working to the degree that MLD patients who receive this therapy are experiencing a dramatic improvement in their disease.
The science is impressive …. and also, the therapy is expensive.
Kevin
Kevin Curran PhD
BridgePoint Bio
I write about the science that is driving our new, advanced medicines. If you find this newsletter interesting…then please hit the link below and share with friends.
BridgePoint Bio
kevin@risingtidebio.com
Sources:
Nathanson, David, and Paul S. Mischel. “Charting the course across the blood-brain barrier.” The Journal of clinical investigation 121.1 (2011): 31-33.
https://www.ema.europa.eu/en/medicines/human/EPAR/libmeldy
*Supporting evidence from clinical info:
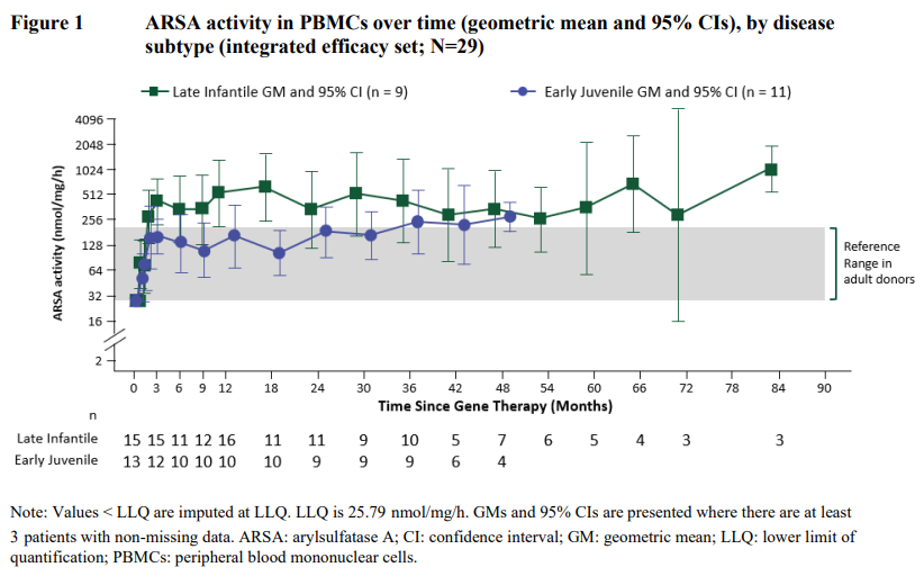
ARSA activity was also measured in cerebrospinal fluid (CSF) as a surrogate compartment of metabolic correction in the brain. The ARSA activity in CSF went from undetectable at Baseline to detectable in all evaluable patients by Month 6 post-treatment and reached reference range levels at Year 1 post-treatment. Thereafter, central reconstitution of ARSA enzymatic activity remained stable within the reference range.
